
This is a fundamental reason for designing reactors with discrete solid fuel separated by moderator, rather than employing a more homogeneous mixture of the two materials. During this slowing-down process it is beneficial to physically separate the neutrons from the uranium, since 238U nuclei have an enormous parasitic affinity for neutrons in this intermediate energy range (a reaction known as "resonance" absorption). This requires the use of a neutron moderator, which absorbs some of the neutrons' kinetic energy, slowing them down to an energy comparable to the thermal energy of the moderator nuclei themselves (leading to the terminology of "thermal neutrons" and "thermal reactors"). The "trick" to making a working reactor is to slow some of the neutrons to the point where their probability of causing nuclear fission in U-235 increases to a level that permits a sustained chain reaction in the uranium as a whole. U-235, on the other hand, can support a self-sustained chain reaction, but due to the low natural abundance of U-235, natural uranium cannot achieve criticality by itself. No amount of U-238 can be made "critical", however, since it will tend to parasitically absorb more neutrons than it releases by the fission process.

U-238 can only be fissioned by neutrons that are fairly energetic, about 1 MeV or above. Natural uranium consists of a mixture of various isotopes, primarily U-238 and a much smaller amount (about 0.72% by weight) of U-235. Due to this fact, in the future the atomic percentage of U235 becomes smaller than 0.72%. The half life of U235 is smaller than the half life of U238 by a 10 factor, approximately. U238 also undergoes spontaneous fission with very low rate, so that the spontaneous fission half life of this nucleus is about 10^15years. Fission reactions typically also release two to four neutrons (depending on the speed on the neutrons inducing the fission and probabilistic. Atomic percentage of U235 and U238 in natural uranium are about 0.72% and 99.28% respectively. For instance uranium-238 decays into thorium-234 with a half-life of almost 4.5 billion years by emitting an alpha particle: 92-uranium-238 > 90-thorium-234 + alpha particle (nucleus of 2-helium-4).
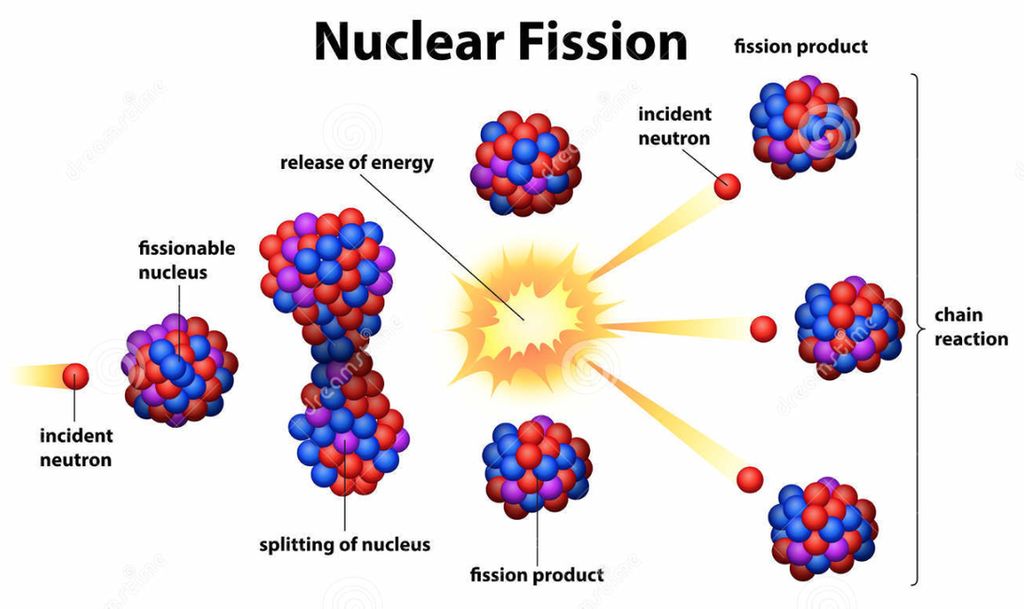
As a result, 235U undergoes fission by the absorption of neutron with very low energy while 238U undergoes fission by the absorption of fast neutron(E_n>0.6 MeV). It is clear that the excitation energy of 236U* is greater than due to pairing energy compared to the excitation energy of 239U* if kinetic energy of absorbed neutrons are the same. Therefore, Formed compound nuclei by absorption of one neutron in 238U and 235U are 239U* and 236U*. Atomic number of both is 92, while neutron numbers of U238 and U235 are 146 and 143, respectively. U235 and U238 are two member of Uranium isotope.


 0 kommentar(er)
0 kommentar(er)
